Mineral classification, the platinum group element mineral is a natural platinum subfamily, including iridium, rhodium, palladium and platinum minerals natural elements of. They have a wide variety of isomorphisms between them, resulting in a series of isomorphous mixed crystals. At the same time, the composition is often mixed with iron , copper , nickel , silver and the like. When their content is high, they constitute the corresponding species. Platinum group elements are all equiaxed crystal systems, single crystals are rare, and occasionally cubic or octahedral fine grains are produced. Generally it is irregular granular, dendritic, grape-like or massive. Color and silver streak to steel gray, metallic luster, opaque, no cleavage, jagged fracture, malleable, electrically conductive and heat long. The metal smelted from the platinum group element mineral is palladium gold, gold, platinum, gold, or the like.
1. Palladium: mainly made from natural smelting. The color is silvery white, the appearance is similar to platinum, and the metallic luster. Hardness 4~4.5. The relative density is 12. The melting point is 1555 ° C. The chemical properties are relatively stable. Because the output is larger than platinum and gold, it is of low value and is rarely used to make jewelry.
2. Sheet metal: It is mainly made of natural enamel and is a rare precious metal. The color is silver white, metallic luster, and opaque. Hard 4~4.5, relative density 12.5. The melting point is high, 1955 ° C. Chemical stability is stable. Because sheet metal is corrosion-resistant and has good gloss, it is mainly used in the electroplating industry. It is plated on other metal surfaces. The coating has a firm color, is not easy to wear, and has good reflection effect.
3. Sheet metal: It is mainly made from natural earthworms or mines. The color is silvery white, with strong metallic luster and hardness of 7. The relative density is 22.40, which is brittle but can be pressed into a foil or drawn into a filament at a high temperature, and has a high melting point of 2454 °C. Chemically stable and insoluble in water. Mainly used in the manufacture of scientific instruments, thermocouples, resistors, etc. High hardness iron and bismuth platinum alloys are commonly used to make nibs and platinum jewelry.
4. Platinum: It is made by melting minerals such as natural platinum and crude platinum ore. Because "Platinum" is a combination of "Gold" and "White", the color is silver white, so it is also called "Platinum". The color is silvery white, metallic luster, hardness 4~4.5, relative density is 21.45. The melting point is high, 1773 ° C. Rich in ductility, it can be drawn into a very fine platinum wire and rolled into a very thin platinum foil. It is extremely stable in chemical properties, insoluble in strong acid and strong in sputum, and does not oxidize in air. Widely used in the jewelry and chemical industries to manufacture advanced chemical vessels, platinum crucibles, and catalysts that accelerate chemical reaction rates.
Second, the type of platinum
1. Pure platinum: the highest quality platinum. Often used to make engagement rings to express the purity of love and forever. In foreign countries, many people think that the use of gold inlaid diamonds may lead to yellowing of the diamonds, thus greatly reducing the price of diamonds. Inlaid with platinum, you can maintain the pure white color of the diamond, especially for engagement rings, set with diamonds in platinum, white and crystal, symbolizing the purity of love forever. However, although platinum is harder than gold, diamonds and jewellery are still not enough. It is often necessary to incorporate *gold to make a platinum alloy for diamonds.
2. Platinum Platinum: An alloy of ruthenium and platinum. The color is also silvery white with a strong metallic luster. Higher hardness. The relative density is also large, chemically determined, and it is an excellent platinum alloy jewelry material. According to the content of hinge and platinum, it can be generally divided into three types: the relative density of the components
10% bismuth-platinum alloy 21.54 1788°C
15% bismuth-platinum alloy 2159 1821°C
5% bismuth-platinum alloy 21.50 1779 ° C
3. K Platinum: A white alloy of gold and other metals. Because platinum is sparsely produced, expensive, and has a high melting point, platinum is rarely used in the general world to produce true K platinum. At present, in order to cater to the needs of consumers for platinum, gold and palladium or nickel, silver, copper, zinc and other metals are used to smelt into a white alloy called "K Platinum". The quality of K Platinum is the same as that of K Gold. The quality of the bundle is 24, and the portion of the mass of gold in it is the K number of K Platinum*. For example, 18K Platinum and 14K Platinum, of which the content of gold is 75% and 58.5%.
Third, platinum jewelry
The understanding and use of platinum by humans is much later than that of gold, which is only about 2,000 years old. According to archaeological data, in the 700 years before BC, the ancient Egyptians had been able to process platinum into platinum crafts with higher craftsmanship. Indians in Central America, before the discovery of the New World in Columbus, also prevailed in platinum ornaments. However, people in other regions knew nothing about platinum until the early 16th century, when the Spanish colonial empire gradually formed, and a large number of Spanish adventurers flocked to Africa and the Americas to explore gold treasure hunt. At the time, when gold was scoured in the rivers of Ecuador, it was repeatedly found that a kind of white metal was mixed in gold, which was actually precious platinum. However, due to the underdeveloped science and low recognition ability, facing the silver-plated platinum, those colonial rulers abandoned it as “inferior broken silverâ€. In 1748, the famous Spanish scientist Anthony Loa discovered the silver-white natural platinum in the Pinto River gold mine. He conducted a careful study and found that the natural platinum is very stable in chemical properties, excellent in ductility and high in melting point. The density is extremely large and is clearly different from metallic silver. Anthony was the first scholar to conduct a detailed study of platinum. In 1780, a skilled craftsman in Paris made a platinum ring, brooch and platinum necklace for King Louis XVI and Queen of France. Therefore, the Louis XVI couple became the first person in the world to have platinum jewelry. Since then, Platinum has gained a great reputation, and it has leapt to the top of the gold jewelry, which is favored by the royal family, the nobles and the wealthy. Since the reserves of platinum in nature are rarer than gold, according to incomplete statistics, the total deposit of platinum in the world is about 14,000 tons (the total reserves of platinum-group mineral resources is about 31,000 tons), although there are more than 60 countries. Platinum crucibles were discovered and mined, but their reserves are highly concentrated in South Africa and the former Soviet Union. Among them, South Africa (Azania) has a platinum reserve of about 12,000 tons. It is the most famous platinum deposit in the world, and is the largest platinum deposit in the world. The former Soviet Union has a platinum reserve of 1,866 tons. It used to be in the Ural sands. Natural platinum with a weight of 8-9 kg was found in the platinum ore, and 427.5 grams of natural platinum was also obtained in the primary strontium. The total reserves of the two accounts for 98% of the world's total reserves. The annual production of platinum in the world is only 85 tons per year, far less than gold. In addition, the melting point of platinum is high. Purifying and smelting platinum is more difficult than gold and consumes more energy. Therefore, its price is more expensive than gold. Platinum gold is elegant and luxurious, symbolizing purity and nobleness. Therefore, people regard it as a token of love and make an engagement ring to express love innocence and forever. If the diamond is set in a silver-plated platinum holder, the crystal diamonds and the brilliant platinum complement each other, bringing out the diamond's whiteness, preciousness and grace.
Platinum jewelry is mainly popular in countries and regions with developed economies such as Europe, America and Japan. Among them, the Japanese are the most favored platinum jewellery, and its sales account for about 75% of the world's platinum jewellery, so it is called "Platinum Power".
China's platinum group elements have few mineral resources, and their reserves are less than 1% of the world's reserves. They can only meet a few percent of demand, mainly for industrial use. The production of jewellery platinum started late, and only a few regions have produced similar products.
Fourth, the identification of platinum and similar metals
Platinum has silver white and streaks, hardness 4~4.5, relative density 21.45, stable chemical properties, insoluble in common acids, and easy to identify with similar metals. Platinum and metal lead and aluminum are deformed in hardness and phase. It can not be restored due to inelastic deformation; and the relative density is much smaller than that of platinum, with lead being 11.36 and aluminum being 2.7, which is about 1/2 and 1/8 of platinum, respectively. Resolve.
Platinum and silver are distinguished by their relative density, hardness, and chemical stability. Although silver is silvery white, the relative density is 10.53, which is only 1/2 of platinum. Moreover, the hardness is low and inelastic. Therefore, the light stroke of the nail can also leave traces. The foil sheet is easy to fold and easy to fold by hand. In the recovery; plus the chemical nature of silver is unstable, dissolved in nitric acid, and released nitrogen dioxide gas. Platinum is insoluble in nitric acid, and it dissolves quickly in heated aqua regia. At room temperature, its dissolution rate is extremely slow, which is generally difficult for the naked eye to detect.
Platinum is a good catalyst and this feature allows rapid identification of platinum. Commonly used hydrogen peroxide reaction method, the specific method is: take a small amount of the sample to be tested, placed in a hydrogen peroxide (H2O2) plastic bottle, if it is platinum, the hydrogen peroxide immediately tumbling and foaming, decompose a large amount of oxygen, the platinum after the reaction is still intact One can also be recycled (it only accelerates decomposition); other white metals such as lead, silver, aluminum, etc. are not affected by this.
A method for separating a platinum group metal of each single crude platinum group metal from a platinum group metal concentrate by a conventional fire method and a hydrometallurgical method.
The traditional method was the main method used by the Soviet Union and the United Kingdom to separate platinum group metals before the 1980s. The technology was kept secret for nearly a hundred years and was not disclosed until the 1960s. The production cycle is long, the process is many, intermittent operation, in the process of repeated smelting, leaching, precipitation, the platinum group metals are not completely separated from each other, a large amount of precious metals are accumulated in the intermediate products, the dispersion loss is large, and the environmental pollution is serious. Since the 1970s, various countries have successively studied and adopted solvent extraction separation methods (see platinum group metal extraction separation), but the traditional methods are also commonly used to process relatively simple raw materials.
The traditional process is complicated, and pyrometallurgy and hydrometallurgy are used interchangeably. It is usually first divided into groups and then separated from each other to obtain a single crude metal or compound. The main separation step comprising roasting leach, aqua regia, platinum, palladium, gold, lead separation, the silver, a molten sodium bisulfate rhodium flooding, a flooding out fusion with sodium peroxide osmium, ruthenium, iridium aqua regia.
Calcination-leaching is an important pretreatment step in the coarse fraction. The purpose is three: 1. The content of base metal in the precious metal concentrate is reduced to less than 1% to reduce its adverse effects on subsequent operations; The grade of precious metal is increased to more than 45%, so as to reduce the processing scale of subsequent operations and reduce the consumption of reagents; 3. Convert the metals such as lanthanum, cerium and lanthanum in the concentrate to the insoluble state of aqua regia to reduce the dissolution of platinum in aqua regia. Co-dissolved and dispersed in palladium and gold. The method is enriched in sulfuric acid roasting in a platinum group metal by a sulfuric acid method. Generally, the concentrate is mixed with a small amount of concentrated sulfuric acid, and calcined in air at a temperature of 773 to 823 K for 2 to 4 hours, and then the base metal sulfate in the calcined material is leached with dilute sulfuric acid to separate it from the platinum group metal. There is a loss of oxidative volatilization during the roasting process. When dilute sulfuric acid is leached, a small amount of palladium, ruthenium, etc. will be dissolved in the leachate, and if necessary, replaced with zinc powder.
The aqua regia dissolves platinum, palladium, gold, and dissolves the concentrate with a portion of nitric acid plus three portions of hydrochloric acid to dissolve the gold, platinum, and palladium into HAuCl 4 , H 2 PtCl 6 , H 2 PdCl 6 , etc., and dissolves. More than 95%. After filtration, most of the lanthanum, cerium and lanthanum remain in the insoluble slag. During the process, a large amount of cerium is oxidized to osmium tetroxide and volatilized in the gas phase. A platinum, palladium, and gold solution containing a small amount of base metal impurities is first boiled to volatilize excess residual acid, and carefully concentrated to a paste. In order to completely destroy the yellow insoluble platinum nitrosyl complex (NO 2 ) 2 PtCl 6 and residual nitric acid, it is necessary to repeatedly add a small amount of concentrated hydrochloric acid to repeatedly evaporate to a paste operation until there is no more NO 2 reddish brown smoke. Gas escapes. The paste is dissolved in water to form a chloride solution containing gold, palladium or platinum. The gold in the solution is first reduced to metal with a reducing agent FeSO 4 or SO 2 and separated from the solution. The FeSO 4 dosage is calculated as follows:
AuCl 3 +3FeSO 4 →Au↓+Fe 2 (SO 4 ) 3 +FeCl 3
After the crude gold is reduced and filtered, the platinum and palladium in the solution are mainly coarsely divided by the difference in solubility between the different valence ammonium salts. That is, the solution is concentrated to 30 to 50 g/L of platinum, and ammonium chloride is added to precipitate platinum (NH 4 ) 2 PtCl 6 having a small solubility, and palladium is formed into a soluble (NH 4 ) 2 PdCl 6 separation. The platinum ammonium salt has the least solubility in a solution containing 17% ammonium chloride, so the amount of ammonium chloride added should be stoichiometric excess over the platinum and palladium ammonium salts. The filtered crude (NH 4 ) 2 PtCl 6 was washed with an ammonium chloride solution and then sent to platinum for refining. The palladium-containing filtrate can be treated in two ways: 1. By introducing chlorine or adding nitric acid, the soluble (NH 4 ) 2 PdCl 6 is oxidized to a poorly soluble (NH 4 ) 2 PdCl 6 precipitate (ie, Pd 2 + is oxidized to Pd 4 ). + ); 2, adding excess ammonia water to neutralize the filtrate to alkaline, so that the palladium forms a soluble colorless dichlorotetraammine palladium complex Pd(NH 3 ) 4 Cl 2 . After separation of the ruthenium metal hydroxide by filtration, the palladium-containing filtrate was neutralized with hydrochloric acid to pH 0.5 to precipitate an egg yellow dichlorodiamine palladium complex Pd(NH 3 ) 4 Cl 2 . The two crude palladium complexes obtained were precipitated and sent to palladium refining. The residual liquid after separating gold, platinum and palladium still contains a small amount of gold and platinum group metals, and is replaced by zinc powder and recovered into the replacement slag. The replacement slag can be returned to the aqua regia.
The separation of lead and silver king water insoluble slag mainly contains silver, bismuth, antimony, bismuth, silicon dioxide, lead and a small amount of other precious metals. Separation of lead and silver has two methods of melting precious lead and direct leaching.
Smelting precious lead usually refers to the lead that is enriched with gold, silver and platinum group metals as precious lead. The insoluble residue was added aqua regia lead oxide or lead carbonate as trapping agent, coke as the reducing agent, sodium carbonate and borax as a flux, expensive output lead smelting reduction at a temperature of 1273 ~ 1373K. After the slag is separated, precious lead water is released and granulated, and lead and silver are dissolved with nitric acid. After filtration, sulfuric acid was precipitated by adding sulfuric acid to the solution. Silver chloride is precipitated by adding hydrochloric acid or sodium chloride to the silver-containing solution after the lead. The precipitated lead sulfate and sodium carbonate solution are boiled and converted into lead carbonate to return smelting precious lead. If the residue of lead nitrate and silver in nitric acid contains gold, platinum or palladium, it can be dissolved again with aqua regia. The insoluble slag mainly contains lanthanum, cerium and lanthanum.
The silver directly leached from the aqua regia slag is present as AgCl, and can be directly leached into a soluble silver ammonia complex by using ammonia containing 1 to 3 mol/L of ammonia. The filtered silver solution was neutralized with hydrochloric acid to precipitate a silver chloride precipitate. The slag after silver separation is leached with ammonium acetate. The filtered lead acetate solution is first boiled to evaporate acetic acid, and then sulfuric acid is added to cause lead to form a slightly soluble lead sulfate precipitate.
The sodium hydrogen sulphate is melted and leached. The slag containing mainly lanthanum, cerium and lanthanum and twice the mass of sodium hydrogen sulphate are mixed and heated to 823-873K for melting. The frit is leached with cold water to sulphuric acid. The filtered barium sulfate solution was neutralized with sodium hydroxide to hydrolyze barium hydroxide. The filtered cesium hydroxide was dissolved in hydrochloric acid and boiled to convert it to chlorodecanoic acid H 3 RhCl 6 to obtain chlorinated acid for refining.
The sodium sulphide is melted in one water and the residue after leaching is mainly composed of strontium, barium and a small amount of strontium. Two times the sodium peroxide of its mass and twice the sodium hydroxide of its mass are added, and the mixture is between 873 and 973K. Melt at temperature. The frit was leached with water to obtain a mixed solution of sodium citrate Na 2 RuO 4 and sodium citrate Na 2 OsO 4 . After filtration, the mash and nail were separately recovered from the solution by oxidative distillation (see 锇钌 extraction separation).
The residue of Wangshui dissolved in water and leaching nails mainly contains strontium. The hydrazine is converted to the IrO 2 state when the sodium peroxide is melted, and can be directly dissolved in aqua regia to obtain a chlorodecanoic acid H 2 IrCl 6 solution. After separating the other elements by the various methods described above, the obtained hydrazine solution is relatively pure, and the mercerized black ammonium chloroantimonate (NH 4 ) 2 IrCl 6 can be precipitated by directly adding ammonium chloride. The obtained ammonium chlorate is sent to refining.
CNC Oscillating Knife Cutting Machine
Concepts:
Oscillating Knife: Oscillating Knife cutting has the advantages of small cutting force, low cutting heat, high surface quality of the workpiece, easy chip handling, improved tool durability, stable processing and high production efficiency.CNC VISION SYSTEM: CCD Vision Cutting system contains two operating modes: direct cutting mode and photo processing mode. Therefore, the processing route manufacturing steps
Differentiate: Direct cutting does not need to draw a photo point, and the tool path file for drawing a photo point can be processed through the buttons on the interface.
INDUSTRIES:
Sign and Graphics, Digital Printing, Packaging, Automotive, Recreational boat, Exhibits, and Fixtures, Sign Making, Gasket Materials, Arts and Crafts, etc.
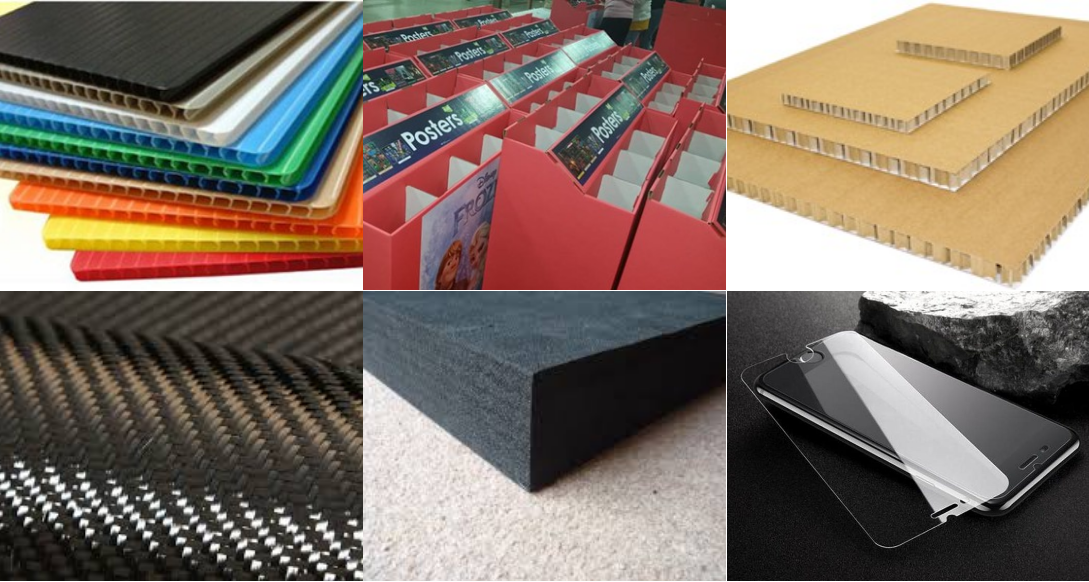
Carpet, Felt, Foam, Closed Cell Foam, Fiberglass Insulation, Composites, Gasket Materials, Rubber, Fabric, Honeycomb Cardboard, Single-ply Cloth/Fabric, Corrugated Cardboard/Paperboard, Vinyl Veneers, Snowboard, Corrugated Plastic, Leather Goods, wood veneer for inlay/marquetry artwork, Carbon fiber pre-preg laminates for aerospace, military, and automotive components i.e.
Why choose Oscillating Knife Cutting Machine
-
Advanced Weihong 53C+TROCEN control system, easy and precise operation;
- The heavy duty thickened bed is welded by square pipe, side hanging
- structure, heavy column, 150*200mm gantry;
- Patent type 1:8 reducer;
- Has the advantages of small cutting force, low cutting heat, high surface quality of the workpiece, easy chip handling, improved tool durability, stable processing and high production efficiency.
- The high precision CNC Oscillating Knife Cutter can induce draft separately and easily cut small pieces.
Main features:
- Working area: Could be customized(1300*2500mm; 1500*3000mm; 2000*3000mm etc.);
- Voltage: 220-420v, 3phase, 50-60hz;
- Spindle:3.5kw air cooling spindle and Oscillating knife+CCD camera;
- X Axis & Y Axis System: Germany ASK/PMT/GC-HIWIN 20 linear guide+Flange slider+Precision 1.25module helical rack+Patent type reducer structure 1:8
- Table: Vacuum & T-slot table;
- Precision machined aluminum parts, using aviation aluminum T6061/6063 material, CNC processing center precision manufacturing.
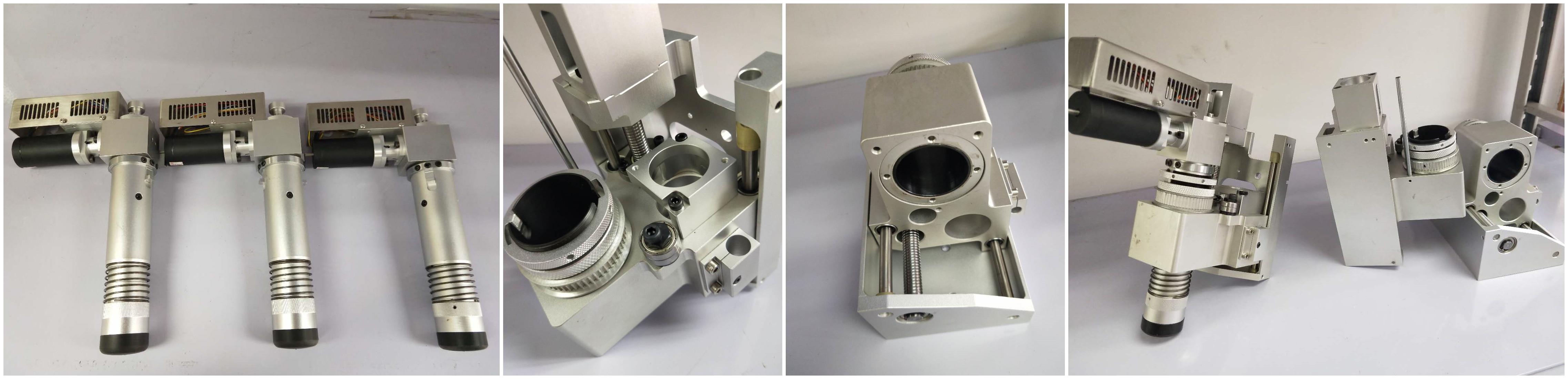


Oscillating Knife Cutter With CCD
Oscillating Knife Cutter With Ccd,Oscillating Knife Cutter,Cnc Router With Oscillating Knife,Oscillating Knife Atc Contour Cutter
Shandong U-May CNC Technology Co., Ltd. , https://www.fiberlasers.de
![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)